Abstract
Background: Tenalisib (RP6530), a highly selective PI3K δ/γ and SIK3 inhibitor has shown promising activity as a single agent in T Cell lymphoma (TCL) and a differentiated safety profile (Huen A et al., Cancers,2020). In vitro studies in TCL cell lines showed synergistic activity when tenalisib was combined with romidepsin. A Phase I/II study of tenalisib in combination with romidepsin was designed to assess safety, pharmacokinetics, and efficacy in patients with relapsed/refractory (R/R) TCL peripheral (PTCL) and cutaneous T cell lymphoma (CTCL) (NCT03770000).
Methods: This was a multi-center, open label study. We performed a Phase I, 3+3 dose escalation study to determine the MTD/recommended Phase II dose (RP2D), and a dose expansion study in both the subtypes separately (PTCL and CTCL). Patients received tenalisib at doses ranging from 400-800 mg BID (fasting), orally in combination with romidepsin at doses ranging from 12-14 mg/m 2, intravenously, given on Days 1,8 and 15 of a 28-day cycle.
Results: Thirty-three patients (16 PTCL and 17 CTCL) who received more than 1 prior therapy were enrolled in the study; 9 in dose escalation and 24 in dose expansion. Of the 33 patients, 64% were refractory to their last therapy. The median number of prior therapies was 3. Most patients (67%) had stage III/IV disease at time of enrolment.
No dose limiting toxicity (DLT) was reported during dose escalation; tenalisib 800 mg BID with romidepsin 14 mg/m 2 (given on Days 1, 8, and 15) was chosen as the RP2D.
The most frequent treatment emergent adverse events (TEAEs) were nausea (All: 73% and ≥G3:0%), thrombocytopenia (All:57% and ≥G3:21%), fatigue (All: 54% and ≥G3:6%), AST elevation (All:33% and ≥G3:6%) ALT elevation (All:27% and ≥G3:18%), neutropenia (All: 27% and ≥G3:15%), vomiting (All:27% and ≥G3:0%), decreased appetite (All: 27% and ≥G3:0%). There were no unexpected TEAEs. Among CTCL patients, five related TEAEs led to drug discontinuation were sepsis, ALT elevation, GGT elevation, rash, and dysgeusia. None of the PTCL patients discontinued the study drug due to related TEAEs. Incidences of TEAEs leading to drug interruption (72%) and dose reduction (45%) of any the drugs in the combination were similar in PTCL and CTCL groups.
Based on C max and AUC, dose proportional exposure of tenalisib was observed from doses 400-800 mg BID. Co-administration of romidepsin with tenalisib did not significantly alter the PK of either agent.
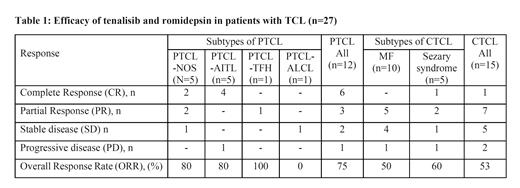
Of the 33 patients enrolled, 27 (12 PTCL and 15 CTCL) who received at least 1 dose of study drug and provided at least 1 post-baseline efficacy assessment were considered evaluable for efficacy as per protocol. The overall response rate (ORR) was of 63%; 7 (26%) patients achieved CR and 10 (37%) patients had PR (Table 1). The median duration of response (DoR) was 5.03 months (range: 2.16 months-Not Reached).
In twelve evaluable PTCL patients, the ORR was 75% with 6 CR (50%) and 3 PR (25%). Among 15 evaluable CTCL patients, 8 responded with an ORR of 53.3%, 1 CR (6.7%) and 7 PR (46.7%). The median DoR was 5.03 (range: 2.16 months-Not Reached) for PTCL and 3.8 months (1.9-18.86) for CTCL. Three of the six (50%) PTCL patients with CR were bridged to transplant.
Six patients who benefitted with the treatment and completed the protocol were enrolled in an open-label compassionate medication study after Cycle 7 and are being followed up.
Conclusions: The combination of tenalisib and romidepsin demonstrates a favorable safety profile and promising anti-tumor activity in patients with R/R TCL. Based on these encouraging results, further development of this combination in PTCL patients in being planned.
Huen: Rhizen: Research Funding; Elorac: Research Funding; Kyowa Kirin: Research Funding; Tillium: Research Funding; Innate: Research Funding; Galderma: Research Funding; Miragen: Research Funding. Ai: Kymria, Kite, ADC Therapeutics, BeiGene: Consultancy. Feldman: Alexion, AstraZeneca Rare Disease: Honoraria, Other: Study investigator. Alderuccio: ADC Therapeutics: Consultancy, Research Funding; Oncinfo / OncLive: Honoraria; Puma Biotechnology: Other: Family member; Inovio Pharmaceuticals: Other: Family member; Agios Pharmaceuticals: Other: Family member; Forma Therapeutics: Other: Family member. Kuzel: Cardinal Health: Membership on an entity's Board of Directors or advisory committees; Exelixis: Membership on an entity's Board of Directors or advisory committees; Genomic Health: Membership on an entity's Board of Directors or advisory committees; Sanofi-Genzyme Genomic Health Tempus laboratories Bristol Meyers Squibb: Honoraria; Abbvie: Other; Curio Science: Membership on an entity's Board of Directors or advisory committees; AmerisourceBergen Corp: Membership on an entity's Board of Directors or advisory committees; CVS: Membership on an entity's Board of Directors or advisory committees; Tempus Laboratories: Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Membership on an entity's Board of Directors or advisory committees; Merck: Other: Data Monitoring Committee Membership; Amgen: Other: Data Monitoring Committee Membership; SeaGen: Other: Data Monitoring Committee Membership; Medpace: Other: Data Monitoring Committee Membership.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal